CHE 011
CHE 011 Homework 6 Name:
You can either type your calculations and answers directly into this document or you can write them out on the piece of paper.
For numerical problems, calculation set up must be shown
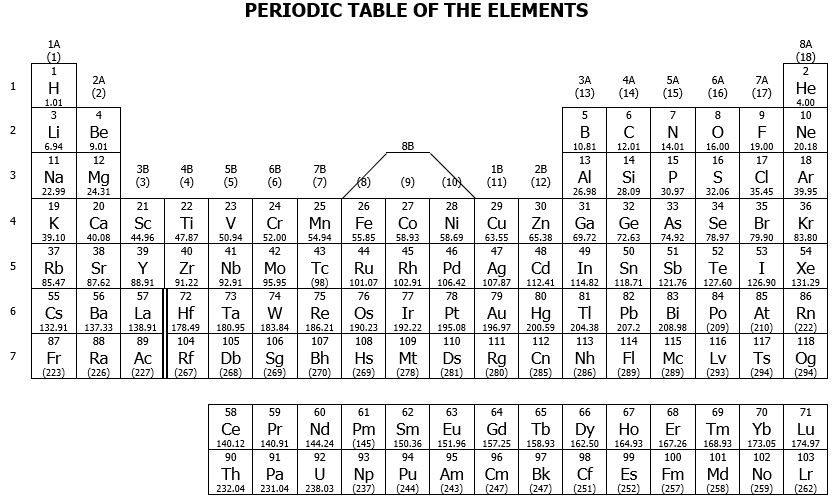
To calculate molar masses, use the values of atomic masses with 2 DP from the Periodic Table provided at the last page.
1. You have a 329.4-gram sample of gold(III) sulfide, Au2S3. After various chemical and physical processes, you are able to separately measure 264.8 grams of gold and 64.6 grams of sulfur.
What are the experimental mass percents of the two elements in this compound?
Answer: %Au = % S =
2. Use molar masses to calculate the theoretical percent of gold and sulfur in gold(III) sulfide, Au2S3.
Answer: %Au = % S =
3. Suppose a certain compound is known to be 47% copper, by mass. How many grams of copper will there be in a 12.5 gram sample of this compound?
Answer:
4. Fill in what items are being counted for each substance. The choices are: molecules, atoms, formula units. Include the substance in your answer.
- 1 mole of silver, Ag = 6.022⨯1023 _____ __________
- 1 mole of methane, CH4 = 6.022⨯1023 ______ __ _____
- 1 mole of chlorine gas, Cl2 = 6.022⨯1023 ______ __ _________
- 1 mole of gold(III) sulfide, Au2S3 = 6.022⨯1023 _______ __________
5. Calculate the molar mass for each of the following substances. Use the Periodic Table provided at the last page. Use these calculated values for questions #6-13 as needed.
a. ammonia, NH3 __
b. glucose, C6H12O6 ____
c. carbon dioxide, CO2 ____
For questions #6-13, fill in the requested information to figure out how to set up the calculation. Then do the calculation a separate sheet of paper and round off the answer to the correct significant figures.
6. How many ammonia molecules are there in 16.78 moles of NH3 ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up:
7. How many moles of ammonia molecules are there in 789 grams of NH3 ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up:
8. How many moles of tin, Sn are needed to give 1.39x1022 tin atoms ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up:
9. How many grams of glucose, C6H12O6 are there in 5.7×1021 glucose molecules ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up:
10. How many moles of chromium are there in 24.61 moles of aluminum dichromate, Al2(Cr2O7)3 ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up:
11. How many grams of carbon dioxide are there in 0.0364 moles of CO2 ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up:
12. How many glucose molecules are there in 7.649 grams of glucose, C6H12O6 ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up:
13. How many oxygen atoms are there in 64 moles of carbon dioxide, CO2 ?
Starting Unit (include substance):
Ending Unit (include substance):
Tool(s) needed:
Answer:
Calculation set up: